- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- Abacavir Sulfate
- 188062-50-2
- C<sub>28</sub>H<sub>38</sub>N<sub>12</sub>O<sub>6</sub>S
- 670.74
- white solid
Your Location:Home > Products > API&Intermediates > Abacavir Sulfate

|
Therapeutic Function |
Antiviral |
|
Biochem/physiol Actions |
Abacavir incorporated in the cells is converted to triphosphate containing guanine analog carbovir (CBV) and it favors the generation of higher double stranded breaks (DSBs). |
|
Synthesis |
Abacavir Sulfate can be prepared by an enantioselective synthesis involving palladium-catalyzed coupling of a chloropurine with a carbocyclic allylic diacetate.Synthesis Step: Treatment of 2,5-diamino-4,6-dihydroxypyrimidine (I) with (chloromethylene)dimethylammonium chloride yielded the dichloropyrimidine with both amino groups derivatized as amidines. Partial hydrolysis with aqueous HCl in hot ethanol gave N-(2-amino-4,6-dichloro-pyrimidin-5-yl)-N,Ndimethylformamidene (II). Subseqent buffered hydrolysis at pH 3.2 yielded the (2-amino-4,6-dichloro-pyrimididin-5-ylamino)acetaldehyde (III). Condensation chloropyrimidine (III) with (1S,4R)-4-amino-2-cyclopentene-1- methanol (IV) in the presence of triethylamine and NaOH gave [2-amino-4- chloro-6-(4-hydroxymethyl-cyclopent-2-enylamino)pyrimidin-5-ylamino]- acetaldehyde (V). The correct enantiomer (IV) of racemic aminocyclopentene was obtained by resolution of diastereomeric salts with D-dibenzoyltartaric acid. Cyclization of (V) to the corresponding purine was accomplished with refluxing triethyl orthoformate or diethoxymethyl acetate to give nucleoside analogue [4-(2-amino-6-chloro-purin-9-yl)-cyclopent-2-enyl]methanol (VI). Displacement of chloride in the purine nucleus with cyclopropyl amine in refluxing butanol afforded abacavir. The structure of obtained compound was confirmed by 1H NMR method and elemental analysis.In practice it is usually used as sulfate salt. |
|
Definition |
ChEBI: Abacavir sulfate is an azaheterocycle sulfate salt that is the sulfate salt of the HIV-1 reverse transcriptase inhibitor abacavir. It is functionally related to an abacavir. |
|
Brand name |
Ziagen (GlaxoSmithKline). |
|
General Description |
Abacavir sulfate belongs to the class of human immunodeficiency virus (HIV) medicines called nucleoside reverse transcriptase inhibitors, with antiretroviral activity against HIV.Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. |
InChI:InChI=1/C14H18N6O.H2O4S/c15-14-18-12(17-9-2-3-9)11-13(19-14)20(7-16-11)10-4-1-8(5-10)6-21;1-5(2,3)4/h1,4,7-10,21H,2-3,5-6H2,(H3,15,17,18,19);(H2,1,2,3,4)/t8-,10-;/m0./s1
The present invention relates to a proce...
Process for removal of the amino protect...
Process for removal of the amino protect...
Process for the preparation of abacavir ...
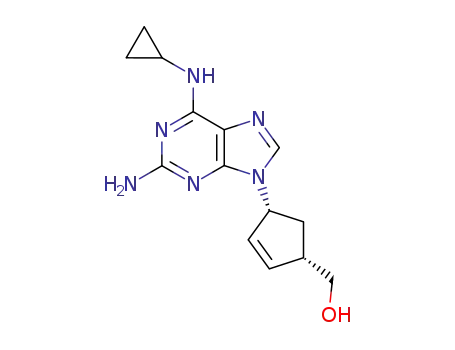
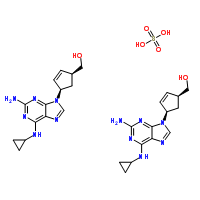
abacavir

![{(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl}methanol sulphate](/upload/2024/12/611b48c0-2681-40d5-be63-df710f299949.png)
{(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl}methanol sulphate
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
In
water; isopropyl alcohol;
at 50 - 55 ℃;
for 2h;
Concentration;
Temperature;
Solvent;
|
95% |
![(-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide](/upload/2024/12/aba4a7ad-45c5-4946-ac54-ed313f034cdd.png)
(-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide

![{(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl}methanol sulphate](/upload/2024/12/611b48c0-2681-40d5-be63-df710f299949.png)
{(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl}methanol sulphate
| Conditions | Yield |
|---|---|
|
(-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide;
With
sodium hydroxide; water; isopropyl alcohol;
for 1h;
Heating / reflux;
With
sulfuric acid;
In
tert-butyl methyl ether;
Product distribution / selectivity;
|
97% |
|
(-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide;
With
isopropyl alcohol; sodium hydroxide;
for 1h;
Reflux;
With
sulfuric acid;
In
tert-butyl methyl ether; isopropyl alcohol;
at 0 - 25 ℃;
Product distribution / selectivity;
|
97% |
|
(-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide;
With
sodium hydroxide; water; isopropyl alcohol;
for 1h;
Heating / reflux;
With
sulfuric acid;
In
tert-butyl methyl ether; water;
at 0 - 5 ℃;
Product distribution / selectivity;
|
97% |
|
(-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide;
With
sodium hydroxide; water; isopropyl alcohol;
for 1h;
Heating / reflux;
With
sulfuric acid;
In
toluene;
Product distribution / selectivity;
|
88% |
|
(-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide;
With
sodium hydroxide; water; isopropyl alcohol;
for 1h;
Heating / reflux;
With
sulfuric acid;
In
water; toluene;
at 0 - 5 ℃;
Product distribution / selectivity;
|
88% |
|
(-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide;
With
sodium hydroxide; water; isopropyl alcohol;
for 1h;
Heating / reflux;
With
sulfuric acid;
In
isopropyl alcohol;
Product distribution / selectivity;
|
79% |
|
(-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide;
With
sodium hydroxide; water; isopropyl alcohol;
With
sulfuric acid;
Product distribution / selectivity;
|
60% |

abacavir
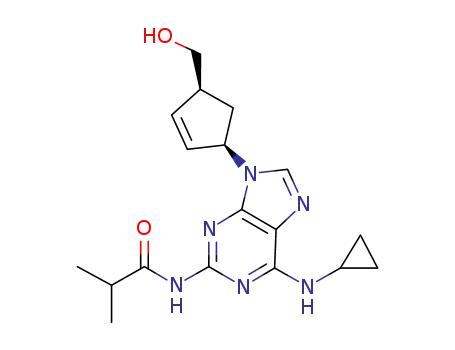
(-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide