- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- Desonide
- 638-94-8
- C<sub>24</sub>H<sub>32</sub>O<sub>6</sub>
- 416.514
- Powder
Your Location:Home > Products > API&Intermediates > Desonide

|
Indications |
Desonide (DesOwen, Tridesilon) is a synthetic corticosteroid. |
|
Manufacturing Process |
Preparation of 11β,21-Dihydroxy-16α,17α-Isopropylidenedioxy-1,4- Pregnadiene-3,20-Dione: A solution of 11β,16α,17α,21-tetrahydroxy-1,4- pregnadiene-3,20-dione (40 mg) in acetone (10 ml) containing hydrochloric acid (three drops; d 1.19) is boiled 3n the steam bath for two minutes and then allowed to stand for eighteen hours at room temperature. The reaction mixture is diluted with water (50 ml) and extracted with chloroform (3x25 ml), the combined extracts then being washed with water (30 ml) and dried over anhydrous sodium sulfate. The residue obtained by removal of solvent crystallized from ethyl acetate-petroleum ether as small plates (25 mg), melting point 257°-260°C. |
|
Therapeutic Function |
Antiinflammatory |
|
Definition |
ChEBI: Triamcinolone acetonide with hydrogen instead of the fluorine substituent at position 9. A corticosteroid anti-inflammatory, it is used topically as a cream, ointment or lotion for the treatment of various skin disorders. |
|
Brand name |
Desowen (Galderma); Tridesilon (Perrigo). |
InChI:InChI=1/C24H32O6/c1-21(2)29-19-10-16-15-6-5-13-9-14(26)7-8-22(13,3)20(15)17(27)11-23(16,4)24(19,30-21)18(28)12-25/h7-9,15-17,19-20,25,27H,5-6,10-12H2,1-4H3/t15-,16-,17-,19+,20+,22-,23-,24+/m0/s1
-
The invention relates to a synthetic met...
The invention discloses a budesonide syn...
Disclosed is a method for preparing a pr...
The present invention discloses a proces...
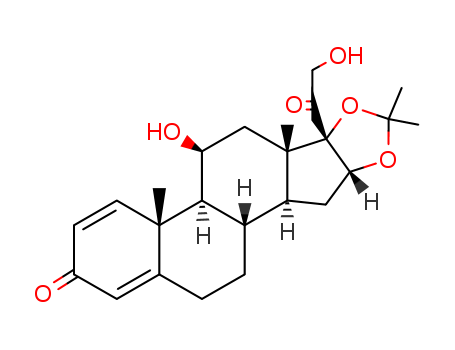
desonide

acetone

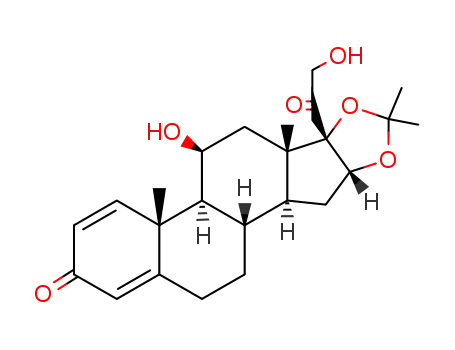
desonide
| Conditions | Yield |
|---|---|
|
With
boron trifluoride;
In
tetrahydrofuran; acetonitrile;
at -5 - 10 ℃;
Inert atmosphere;
|
85% |
|
With
methanesulfonic acid;
at -10 ℃;
for 0.133333h;
Temperature;
|
C26H32O7


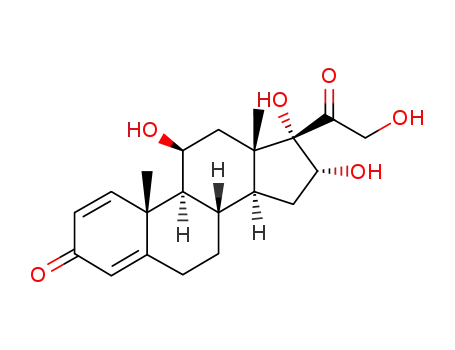
desonide
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
methanol;
at 30 ℃;
for 0.666667h;
Temperature;
Inert atmosphere;
Large scale;
|
81% |

desonide
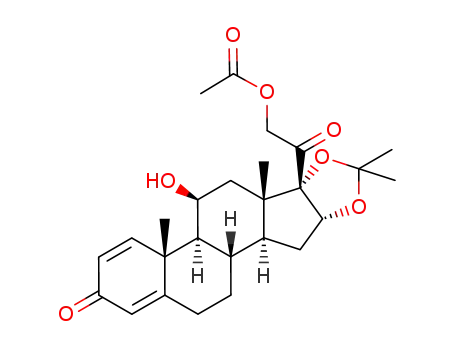
11β,16α,17α,21-tetrahydroxy-1,4-pregnadiene-3,20-dione 16,17-acetonide 21-acetate
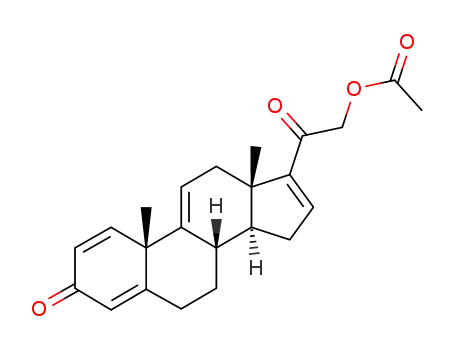
2-((10S,13S,14S)-10,13-dimethyl-3-oxo-6,7,8,10,12,13,14,15-octahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl acetate
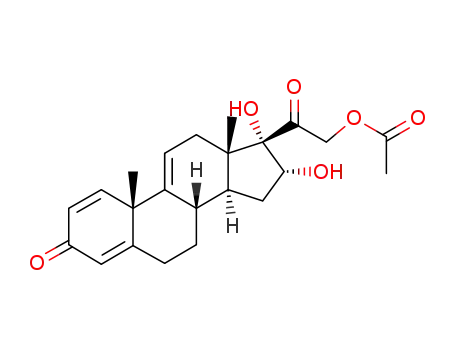
16α,17α,21-trihydroxypregna-1,4,9(11)-triene-3,20-dione-21-acetate
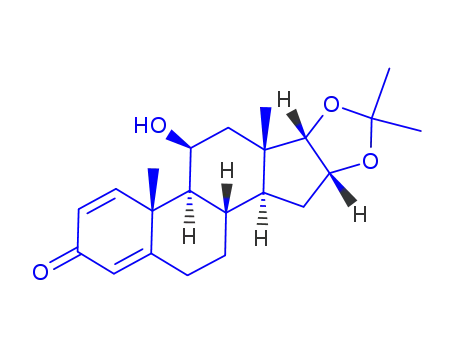
11β-hydroxy-16α,17α-<(1-methylethylidene)bis(oxy)>-3-oxoandrosta-1,4-diene
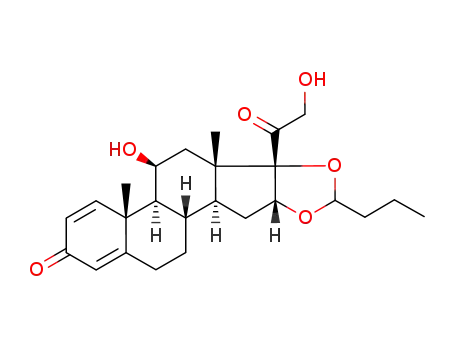
budesonide
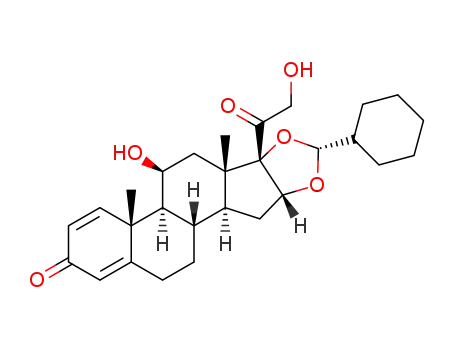
desisobutyryl ciclesonide
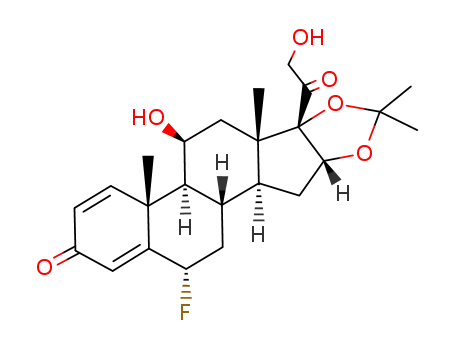
flunisolide