- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- Cyproterone acetate
- 427-51-0
- C<sub>24</sub>H<sub>29</sub>ClO<sub>4</sub>
- 416.945
- Crystalline solid
Your Location:Home > Products > API&Intermediates > Cyproterone acetate

|
Indications |
Cyproterone acetate is a progestational antiandrogen that blocks androgen receptor binding and suppresses androgen-sensitive tissues. It is available in a topical form in Europe for the treatment of hirsutism. |
|
Manufacturing Process |
2.34 g of 1,2α-methylene-δ4,6-pregnadiene-17α-ol-3,20-dione-17-acetate are dissolved in 18.25 cc of ethylene chloride which contains 844 rng of perbenzoic acid. The solution is stored for 16 hours at +5°C and 7 hours at room temperature. It is then diluted with methylene chloride and, with aqueous ferrous sulfate solution, sodium bicarbonate solution and with water washed until neutral.The organic phase is dried over sodium sulfate and then concentrated to dryness. 1.62 g of the thus obtained crude 1,2α-methylene-6,7α-oxido-δ4- pregnene-17α-ol-3,20-dione-17-acetate are dissolved in 109 cc of glacial acetic acid. This solution is then saturated at room temperature with hydrogen chloride gas and stored for 20 hours, It is then diluted with methylene chloride and washed with water until neutral.The organic phase is dried over sodium sulfate and then concentrated to dryness. The thus obtained crude 6-chloro-1α-chloromethyl-δ4,6-pregnadiene17α-ol-3,20-dione-17-acetate is heated to boiling in 20 cc of collidine for 20 minutes under nitrogen. After dilution with ether it is washed with 4 N hydrochloric acid and washed with water until neutral.After drying over sodium sulfate and concentration to vacuum the remaining residue is subjected to chromatography over silica gel. Using a benzene-ethyl acetate mixture (19:1) there is eluated 900 mg of 6-chloro-1,2α-methyleneδ4,6-pregnadiene-17α-ol-3,20-dione-17-acetate, which upon recrystallization from isopropyl ether melts at 200° to 201°C. |
|
Therapeutic Function |
Antiandrogen |
|
Drug interactions |
Potentially hazardous interactions with other drugs None known |
|
Metabolism |
Cyproterone is metabolised by various pathways including hydroxylation and conjugation; about 35% of a dose is excreted in urine, the remainder being excreted in the bile. The principal metabolite, 15β-hydroxycyproterone, has anti-androgenic activity |
InChI:InChI=1/C24H29ClO4/c1-12(26)24(29-13(2)27)8-6-16-14-10-20(25)19-11-21(28)15-9-18(15)23(19,4)17(14)5-7-22(16,24)3/h10-11,14-18H,5-9H2,1-4H3/t14?,15-,16?,17?,18+,22+,23+,24+/m1/s1
The invention discloses a cyproterone ac...
The invention relates to a preparation p...
The present invention relates to improve...
-
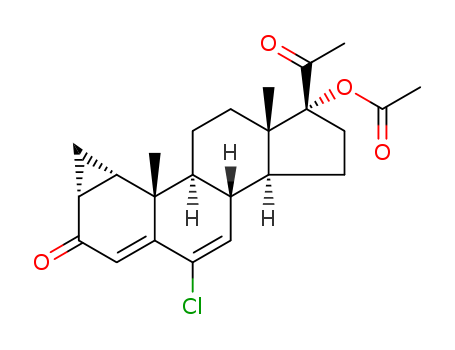
6-chloro-17a-acetoxy-pregnane-1,4,6-,triene-3,20-dione

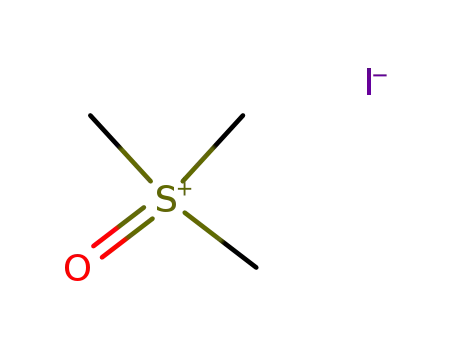
trimethylsulfoxonium iodide

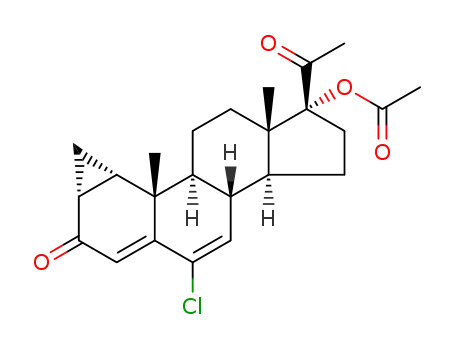
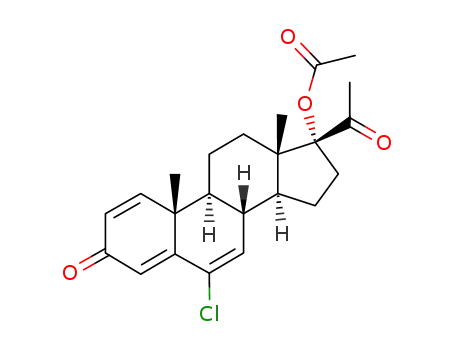
cyproterone acetate
| Conditions | Yield |
|---|---|
|
6-chloro-17a-acetoxy-pregnane-1,4,6-,triene-3,20-dione; trimethylsulfoxonium iodide;
With
sodium hydride;
In
dimethyl sulfoxide;
at 5 - 20 ℃;
for 29.5h;
With
hydrogenchloride;
In
water; dimethyl sulfoxide;
at 0 ℃;
|
51.51% |
C24H30Cl2O4


cyproterone acetate
| Conditions | Yield |
|---|---|
|
With
sodium carbonate;
In
methanol; N,N-dimethyl-formamide;
at 50 - 55 ℃;
for 5h;
|

6-chloro-17a-acetoxy-pregnane-1,4,6-,triene-3,20-dione

trimethylsulfoxonium iodide
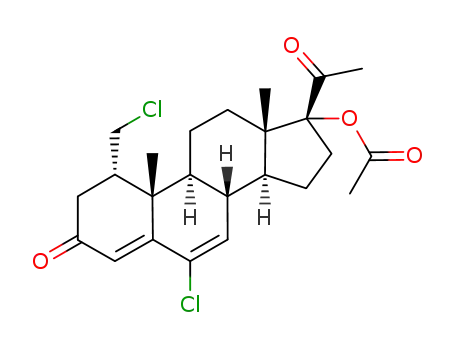
6-chloro-1α-chloromethyl-Δ4,6-pregnadien-17α-ol-3,20-dione-17-acetate
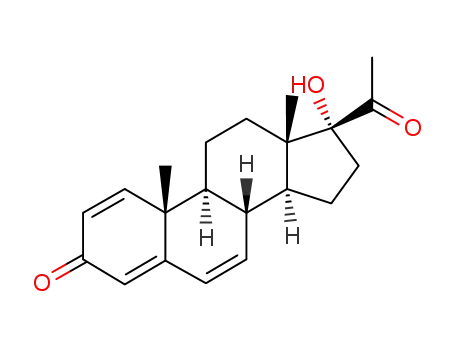
1,4,6-triene-3,20-dione-17α-hydroxyprogesterone
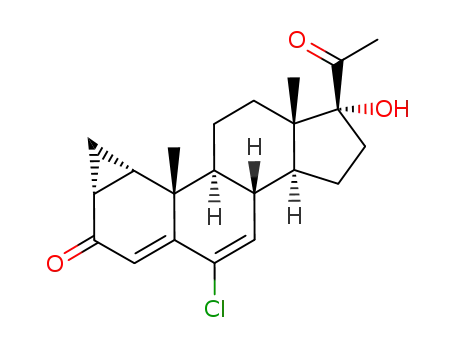
cyproterone