- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
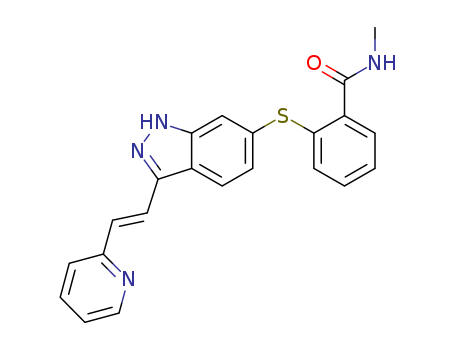
- Axitinib
- 319460-85-0
- C<sub>22</sub>H<sub>18</sub>N<sub>4</sub>OS
- 386.477
- Off-white solid
Your Location:Home > Products > API&Intermediates > Axitinib

|
FDA approved axitinib use of treating advanced kidney cancer |
January 27, 2012, the FDA approved axitinib for the treatment of advanced kidney cancer (renal cell carcinoma) which other drugs unanswer . Inlyta is manufactured and sold by Pfizer,and is a oral pill taken twice a day. Renal cell carcinoma is a type of tumor originating from the tubular endothelial cells. Axitinib can prevent certain protein called kinases playing a role in tumor growth and metastasis . Axitinib is a small molecule tyrosine kinase inhibitor, effective against multiple targets, including VEGF receptors 1, 2 and 3. Dr. Richard Pazdur, hematology and oncology drugs office director of FDA Drug Evaluation and Research Centre, said: "This is the seven kind of drugs allowed treating metastatic or advanced renal cell carcinoma since 2005 . Overall, during this time ,record level of drug development has dramatically changed the treatment of metastatic renal cell carcinoma paradigm, and offers a variety of treatment options for patients. " In recent years, the drug has been approved for the treatment of kidney cancer include sorafenib (2005), sunitinib (2006), temsirolimus(2007), everolimus (2009), bevacizumab(2009) and pazopanib(2009). The above information is edited by the lookchem of Tian Ye. |
|
Biochem/physiol Actions |
Axitinib (AG-013736) is an orally available, potent (picomolar) and selective tyrosine kinase inhibitor that blocks VEGF receptors 1, 2 and 3. The drug blocks VEGF-mediated endothelial cell survival, tube formation, and downstream signaling through endothelial nitric oxide synthase, Akt and extracellular signal-regulated kinase. |
|
Synthesis |
Numerous patents and papers have been disclosed on the synthesis of axitinib, a recently published manuscript details the development of the manufacturing route, and this route is depicted in the scheme. The synthesis began with Migita coupling of commercial iodide 17 with thiophenol 18. Interestingly, this transformation’s efficiency relied upon attention to the number of equivalents of base and an inert atmosphere in the reaction vessel, conditions which minimized catalyst poisoning during the reaction. Without isolation, indazole 19 was iodinated to afford diarylthioether 20 in 85-90% yield over the two steps. Protection of the indazole within 20 as its acetamide preceeded a Heck reaction with 2-vinylpyridine, and then subsequent removal of the indazole protection followed by a series of recrystallizations yielded axitinib (IV) in a combined 62% yield over the final 4 steps. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antipsychotics: avoid with clozapine (increased risk of agranulocytosis); avoid with pimozide. Concomitant use with strong CYP3A4/5 inhibitors: avoid; however, if concomitant use cannot be avoided then reduce the dose of axitinib by approximately half; subsequent doses can be increased or decreased based on individual safety and tolerability; if CYP3A4/5 inhibitor is discontinued, then increase the axitinib dose used prior to initiation of the strong inhibitor after 3-5 half-lives of the inhibitor (strong CYP3A4/5 inhibitors include ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, ritonavir, saquinavir, and voriconazole). |
|
Metabolism |
Axitinib is metabolised primarily in the liver by CYP3A4/5 and to a lesser extent by CYP1A2, CYP2C19, and UGT1A1. Most of the drug is excreted via the faeces and urine as metabolites. |
|
Definition |
ChEBI: An indazole substituted at position 3 by a 2-(pyridin-2-yl)vinyl group and at position 6 by a 2-(N-methylaminocarboxy)phenylsulfanyl group. Used for the treatment of advanced renal cell carcinoma after failure of a first line systemic tr atment. |
|
Brand name |
Inlyta |
InChI:InChI=1/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+
The goal of photopharmacology is to deve...
1) A process for preparing Axitinib ( N-...
In this study, we aimed at the applicati...
The present invention relates to a water...
A mechanically-activated chemoselective ...
C22H18N4OS*C4H4O4

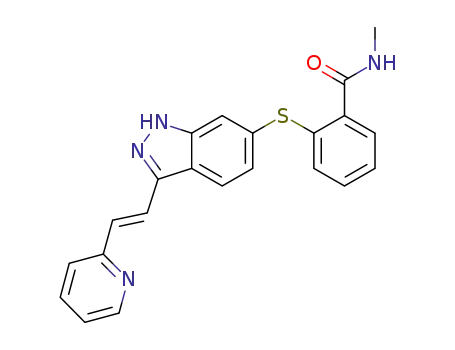
axitinib
| Conditions | Yield |
|---|---|
|
With
1,2-diaminopropan;
In
1-methyl-pyrrolidin-2-one;
at 40 - 70 ℃;
for 1h;
|
95% |
![(E)-N-methyl-2-{[3-(2-(pyridin-2-yl)ethenyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-indazol-6-yl]thio}benzamide](/upload/2024/12/65aade91-0cd0-4929-94b0-80dc215f46ff.png)
(E)-N-methyl-2-{[3-(2-(pyridin-2-yl)ethenyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-indazol-6-yl]thio}benzamide


axitinib
| Conditions | Yield |
|---|---|
|
With
toluene-4-sulfonic acid;
In
neat (no solvent);
for 0.75h;
Milling;
|
99% |
|
With
toluene-4-sulfonic acid;
In
methanol; water;
at 64 ℃;
for 1 - 5h;
Product distribution / selectivity;
|
98% |
|
With
toluene-4-sulfonic acid;
In
methanol; water;
at 65 ℃;
for 4h;
Reagent/catalyst;
Inert atmosphere;
|
95.4% |
|
With
toluene-4-sulfonic acid;
In
methanol;
for 4h;
Reflux;
|
95.1% |
|
With
toluene-4-sulfonic acid;
In
methanol;
at 65 ℃;
for 4h;
Product distribution / selectivity;
Heating / reflux;
|
92.5% |
|
(E)-N-methyl-2-{[3-(2-(pyridin-2-yl)ethenyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-indazol-6-yl]thio}benzamide;
With
toluene-4-sulfonic acid;
In
methanol;
for 4h;
Heating / reflux;
With
sodium hydrogencarbonate;
In
water; ethyl acetate;
at 20 ℃;
for 2h;
|
92.5% |
|
With
hydrogenchloride; methanol; water;
at 60 ℃;
for 6h;
|
326.4 g |
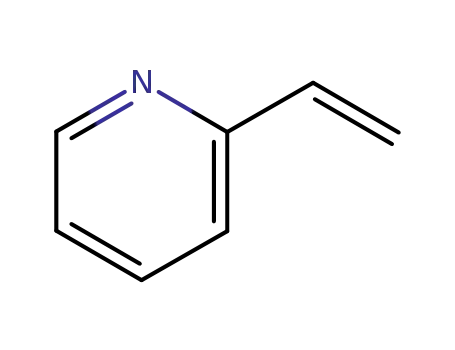
2-vinylpyridine
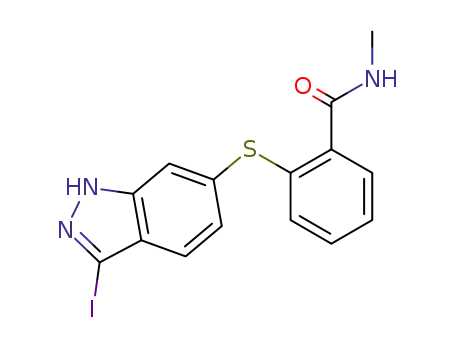
2-((3-iodo-1H-indazol-6-yl)thio)-N-methylbenzamide
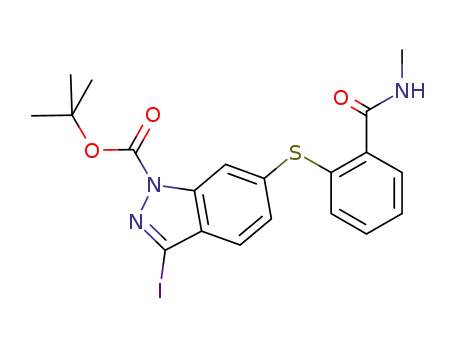
N-1 Boc 2-(3-iodo-1H-indazol-6-ylsulfanyl)-N-methyl-benzamide
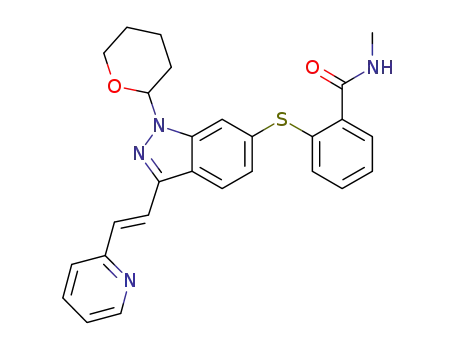
(E)-N-methyl-2-{[3-(2-(pyridin-2-yl)ethenyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-indazol-6-yl]thio}benzamide
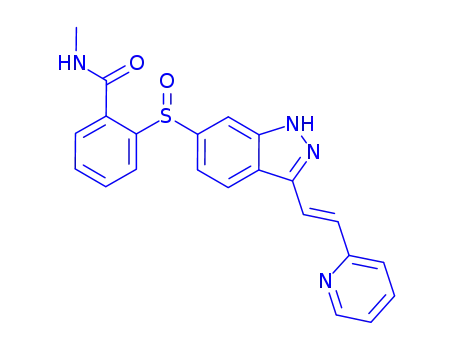
Axitinib sulfoxide