- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
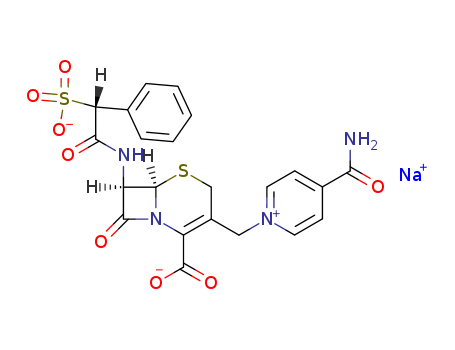
- Cefsulodine sodium
- 52152-93-9
- C<sub>22</sub>H<sub>19</sub>N<sub>4</sub>NaO<sub>8</sub>S<sub>2</sub>
- 554.537
- White to pale yellow crystalline powder
Your Location:Home > Products > API&Intermediates > Cefsulodine sodium

|
Cephalosporins |
Cefsulodine sodium is a cephalosporin antibiotic, its antimicrobial spectrum is narrow, because it has good permeability to the outer layer of the cell wall of Pseudomonas aeruginosa , and it has a high affinity on penicillin-binding protein LA, LB and Ⅲ and thus it has strong bactericidal inhibition effect on the formation of the cell wall peptidoglycan , it has a strong and specific bactericidal effect on P. aeruginosa , the MIC is 1.5μg/ml, and it has high stability on β-lactamase produced by itself . Its antimicrobial activity against Pseudomonas aeruginosa is stronger than cefoperazone, methyl benzyl amoxicillin (10 times), carbenicillin, piperacillin and hydroxyl ampicillin (16 to 32-fold) , and its antimicrobial activity against Pseudomonas aeruginosa is equal to aminoglycoside antibiotics such as gentamicin, dibekacin . And there is no cross-resistance.After intramuscular or intravenous administration 0.5g, 15min respectively ,it achieves the maximum blood concentration of 37.2μg/ml, and the concentration of the drug increases with the increase of the dose . It can be quickly distributed to the kidneys, blood plasma, lung, heart, digestive tract, liver and spleen, and it can go access to sputum, wound exudate, amniotic fluid and breast milk. PBP is 70%, t1/2 is 1.5h, it is mainly excreted in the urine, 6h after treatment the urine excretion rate is 60% to 70%, metabolites with antibacterial activity are not found in the urine . In renal dysfunction patients , plasma concentration increases, half-life extends and urinary excretion rate decreases. Sodium cefsulodin is clinically used for the treatment of respiratory tract infections caused by Pseudomonas aeruginosa, pyelonephritis, cystitis, peritonitis, septicemia, prostatitis, burns, wounds and otitis media secondary infection, corneal ulcers, especially penicillin and aminoglycosides antibiotic treatment ineffective Pseudomonas aeruginosa infection. Adverse reactions occurring rate is approximately 1.3%, it is similar to other cephalosporins. Occasionally allergic reactions occur, such as rash, urticaria.There are gastrointestinal reactions. Injection site is painful, intravenous injection causes phlebitis, mildly elevated serum transaminases , few visible proteinuria; there are also visible thrombocytopenia, increased eosinophils; rare thrombocytopenia, vitamin deficiency and the like. Patients allergic to the chemicalsare are banned .Patients allergic to penicillin,and cephalosporin , having history of allergy diseases, allergic bodies, severe renal dysfunction, and pregnant women should use with caution. Cefsulodine sodium together with diuretics, aminoglycosides can increase renal toxicity. Check liver function, kidney function and blood at regular intervals . The above information is edited by the lookchem of Tian Ye. |
|
Toxicity grading |
Low toxicity |
|
Acute toxicity |
Intravenous-rat LD50: 3030 mg/kg; Intravenous-Mouse LD50: 3780 mg/kg. |
|
Flammability and hazard characteristics |
Thermal decomposition produces toxic nitrogen oxides, sulfur oxides, sodium oxide fumes. |
|
Storage Characteristics |
Ventilated, low-temperature ,dry storeroom. |
|
Extinguishing agent |
Water, carbon dioxide, foam, powder. |
|
Manufacturing Process |
0.514 g (4 x 10-3 mol) of 7-(α-sulfophenylacetamido)cephalosporanic acid, 0.466 g (3 x 10-3 mol) of isonicotinamide and 2.0 g (2.06 x 10-3 mol) of potassium thiocyanate were dissolved in 2.5 ml of water. The resulting solution was allowed to stand and heated for 20 hours in a thermostat kept at 50°C and then directly purified by chromatography on an Amberlite XAD-2 column (16 x 880 mm). Subsequently, the fractions containing the cephalosporins were collected and subjected to freeze-drying to obtain 270 g of the title product in the form of a pale yellowish white powder. The product is usually used as the sodium salt. |
|
Therapeutic Function |
Antibiotic |
|
Biological Activity |
cefsulodin, formerly named as sce-129, is a cephalosporin with a spectrum of antibacterial activity against staphylococcus aureus, pseudomonas aeruginosa, and most other gram-positive cocci [1]. cefsulodin shows little activity against other species of acinetobacter spp., pseudomonas, or members of the enterobacteriaceae [1]. cefsulodin is a β-lactam antibiotic that involved in lysing actively-growing e. coli by specifically binding to the intermembrane proteins, penicillin-binding proteins 1a and b, whose transglycosylase and transpeptidase activities are involved in cell elongation and septation [2].cefsulodin was active against p. aeruginos. cefsulodin was active against penicillinase-producing strains of s. aureus. the mics of cefsulodin for pseudomonas aeruginosa and its mutants pseudomonas aeruginosa pao4089 were 0·78 and 12· mg/l [3]. cefsulodin was active in minimum inhibitory concentrations (mics) of 0.5 to 64 μg/ml. cefsulodin was active against p. diminuta, p. maltophilia, p. paucimobilis, and p. pseudoalcaligenes with mics of 1-32 μg/ml. cefsulodin was not hydrolyzed by the β-lactamase induced in p. aeruginosa by growth in the presence of benzylpenicillin and was a poor substrate for β-lactamases from enterobacter cloacae and proteus morganii [4]. |
|
references |
[1] barry a l, jones r n, thornsberry c. cefsulodin: antibacterial activity and tentative interpretive zone standards for the disk susceptibility test[j]. antimicrobial agents and chemotherapy, 1981, 20(4): 525-529.[2] jacoby g h, young k d. cell cycle-independent lysis of escherichia coli by cefsulodin, an inhibitor of penicillin-binding proteins 1a and 1b[j]. journal of bacteriology, 1991, 173(1): 1-5.[3] bryan l e, kwan s, godfrey a j. resistance of pseudomonas aeruginosa mutants with altered control of chromosomal beta-lactamase to piperacillin, ceftazidime, and cefsulodin[j]. antimicrobial agents and chemotherapy, 1984, 25(3): 382-384.[4] king a, shannon k, phillips i. in vitro antibacterial activity and susceptibility of cefsulodin, an antipseudomonal cephalosporin, to beta-lactamases[j]. antimicrobial agents and chemotherapy, 1980, 17(2): 165-169. |
|
Category |
Toxic substances |
InChI:InChI=1/C22H20N4O8S2.Na/c23-18(27)13-6-8-25(9-7-13)10-14-11-35-21-15(20(29)26(21)16(14)22(30)31)24-19(28)17(36(32,33)34)12-4-2-1-3-5-12;/h1-9,15,17,21H,10-11H2,(H4-,23,24,27,28,30,31,32,33,34);/q;+1/p-1
The invention relates to the technical f...
The invention is directed to an improved...
The invention is directed to an improved...
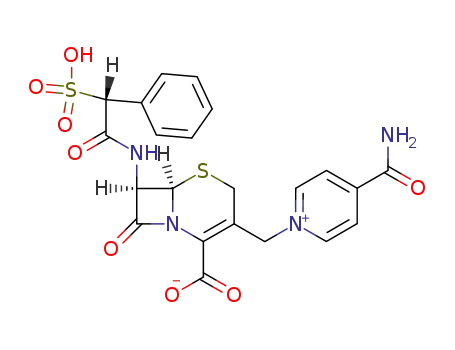
cefsulodin

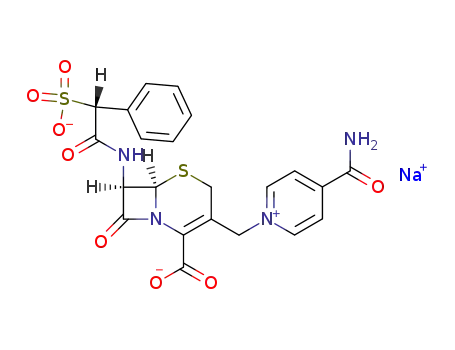
Cefomonil
| Conditions | Yield |
|---|---|
|
With
sodium acetate;
In
ethanol; water;
pH=4 - 4.25;
Product distribution / selectivity;
|
85% |
|
With
sodium acetate; pyrographite;
In
water;
pH=4.1;
Large scale;
|
4.9 kg |
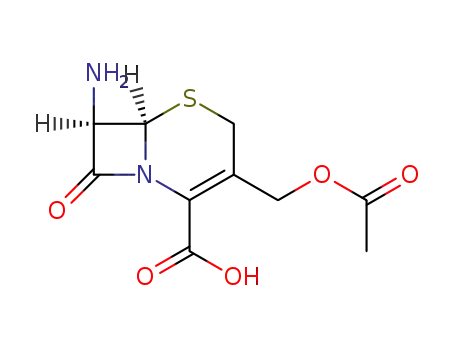
7-Aminocephalosporanic acid


Cefomonil
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: 1,1,1,3,3,3-hexamethyl-disilazane; sulfuric acid / dichloromethane / 4 h / Reflux; Large scale
1.2: Large scale
2.1: sodium hydrogencarbonate / dichloromethane; water / 1 h / 0 - 5 °C / Large scale
3.1: sodium acetate; pyrographite / water / pH 4.1 / Large scale
With
sulfuric acid; sodium acetate; sodium hydrogencarbonate; pyrographite; 1,1,1,3,3,3-hexamethyl-disilazane;
In
dichloromethane; water;
|

cefsulodin

7-Aminocephalosporanic acid
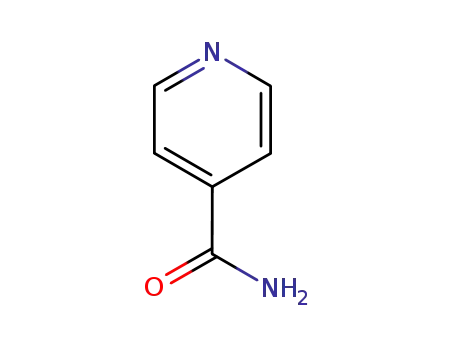
isonicotinamide
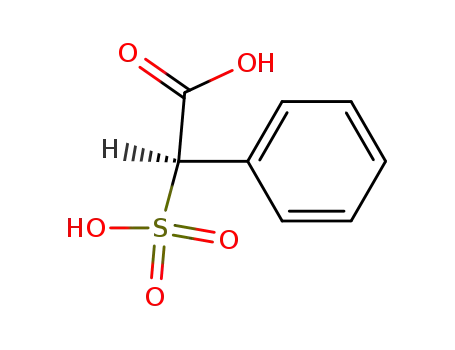
2-phenyl-2-sulfoacetic acid