- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- Eltrombopag olamine
- 496775-62-3
- C25H22N4O4·2(C2H7NO)
- 564.6394
Your Location:Home > Products > API&Intermediates > Eltrombopag olamine
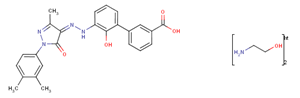

|
Synthesis |
The synthesis began with the nitration of 2-bromophenol (39) with sodium nitrate and sulfuric acid in water at 10°C to give 2-bromo-6-nitrophenol (40) in 25% yield, which was methylated using methyl iodide and potassium carbonate in refluxing acetone providing 2-bromo- 6-nitroanisole (41) in 76% yield (the Scheme).40 Suzuki coupling of compound 41 with 3-carboxyphenyl boronic acid with Pd(PPh3)4 and 2 M sodium carbonate in refluxing dioxane gave 20-methoxy- 30-nitrobiphenyl-3-carboxylic acid (42) in 47% yield as a tan powder. Demethylation using 48% HBr (aq) in refluxing acetic acid resulted in a 79% yield of 20-hydroxy-30-nitrobiphenyl-3-carboxylic acid (43). The nitro group of compound 43 was reduced via catalytic hydrogenation at 50 psi at room temperature over Pd/C in mixed ethanol/3 M aq NaOH solution to give 30-amino-20-hydroxybiphenyl- 3-carboxylic acid (44) in quantitative yield. The intermediate 1-(3,4-dimethylphenyl)-3-methyl-2,5-dihydro-1Hpyrazol- 5-one (47) was prepared by condensing of 3,4-dimethylphenyl- hydrazine 45 with ethyl acetoacetate 46 with sodium acetate in refluxing acetic acid in 76% yield. Treatment of (44) with sodium nitrite in 1 M HCl at 5°C, followed by condensation with 1-(3,4-dimethylphenyl)-3-methyl-2,5-dihydro-1H-pyrazol-5-one (47) at a constant pH of 7–8 via the addition of sodium bicarbonate and ethanol afforded eltrombopag in 32% yield. Finally, eltrombopag was treated with hydroxyl ethylamine to give eltrombopag olamine (VIII). |
InChI:InChI=1/C25H22N4O4.2C2H7NO/c1-14-10-11-19(12-15(14)2)29-24(31)22(16(3)28-29)27-26-21-9-5-8-20(23(21)30)17-6-4-7-18(13-17)25(32)33;2*3-1-2-4/h4-13,26,30H,1-3H3,(H,32,33);2*4H,1-3H2/b27-22-;;