- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- Fluvoxamine maleate
- 61718-82-9
- C<sub>15</sub>H<sub>21</sub>F<sub>3</sub>N<sub>2</sub>O<sub>2</sub><sup>.</sup>C<sub>4</sub>H<sub>4</sub>O<sub>4</sub>
- 434.413
- white crystalline powder
Your Location:Home > Products > API&Intermediates > Fluvoxamine maleate

|
Therapeutic Function |
Antidepressant |
|
Biological Activity |
Selective serotonin reuptake inhibitor; antidepressant. Binds to the human 5-HT transporter with a K i of 1.6 nmol/l. Also available as part of the Serotonin Uptake Inhibitor Tocriset? . |
|
Biochem/physiol Actions |
Fluvoxamine maleate is a selective neuronal serotonin reuptake inhibitor. It functions as an antidepressant and anti-obsessive agent. It is useful in treating obsessive compulsive disorder and panic disorder. |
|
Veterinary Drugs and Treatments |
Fluvoxamine may be considered for use in treating a variety of behavior- related diagnoses in dogs and cats, including aggression and stereotypic behaviors (and other obsessive-compulsive behaviors). |
|
Drug interactions |
Potentially hazardous interactions with other drugsAminophylline and theophylline: increased aminophylline and theophylline concentrations - avoid; if not possible, halve aminophylline or theophylline dose and monitor levels.Analgesics: increased risk of bleeding with aspirin and NSAIDs; risk of CNS toxicity increased with tramadol; concentration of methadone possibly increased.Anti-arrhythmics: increased risk of toxicity with mexiletine.Anticoagulants: effect of coumarins possibly enhanced; possibly increased risk of bleeding with dabigatran.Antidepressants: avoid with reboxetine, MAOIs, moclobemide and St John’s wort; possibly enhanced serotonergic effects with mirtazapine; fluvoxamine inhibits metabolism of duloxetine - avoid; can increase tricyclics concentration; metabolism of agomelatine reduced; possible increased risk of convulsions with vortioxetine.Antiepileptics: antagonise anticonvulsant threshold; concentration of carbamazepine, fosphenytoin and phenytoin increased.Antimalarials: avoid with artemether/lumefantrine and piperaquine with artenimol.Antipsychotics: concentration of asenapine, haloperidol, clozapine and olanzapine increased; increased risk of ventricular arrhythmias with droperidol and possibly pimozide - avoid.Antivirals: concentration possibly increased by ritonavir.Ciclosporin: may increase ciclosporin concentration.Clopidogrel: possibly reduced antiplatelet effect.Cytotoxics: concentration of pomalidomide increasedDapoxetine: possible increased risk of serotonergic effects - avoid.Dopaminergics: increased risk of CNS toxicity with rasagiline - avoid; hypertension and CNS excitation with selegiline - avoid. 5HT1 agonists: risk of CNS toxicity increased with sumatriptan; possibly increased risk of serotonergic effects with naratriptan; inhibits metabolism of frovatriptan; possibly inhibits metabolism of zolmitriptan - reduce zolmitriptan dose.Linezolid: use with care, possibly increased risk of side effects.Lithium: increased risk of CNS effects - monitor levels.Melatonin: concentration of melatonin increased - avoid.Methylthioninium: risk of CNS toxicity - avoid if possible.Muscle relaxants: increased risk of toxicity with tizanidine - avoidPirfenidone: concentration of pirfenidone increased - avoid. |
|
Metabolism |
Fluvoxamine undergoes extensive hepatic transformation by CYP2D6, mainly via oxidative demethylation, into at least 9 metabolites. The 2 major metabolites showed negligible pharmacological activity. The other metabolites are not expected to be pharmacologically active.Excretion is mainly in the urine; about 2% of a dose is excreted as unchanged drug. |
|
Brand name |
Luvox (Solvay Pharmaceuticals);FLOXYFRAL. |
|
General Description |
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards |
InChI:InChI=1/C15H21F3N2O2.C4H4O4/c1-21-10-3-2-4-14(20-22-11-9-19)12-5-7-13(8-6-12)15(16,17)18;5-3(6)1-2-4(7)8/h5-8H,2-4,9-11,19H2,1H3;1-2H,(H,5,6)(H,7,8)/b20-14+;2-1-
The present invention relates to an indu...
A process is described for the preparati...
![5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan-1-one](/upload/2024/12/8e1163b3-008d-4a56-b214-15ae07cf771c.png)
5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan-1-one

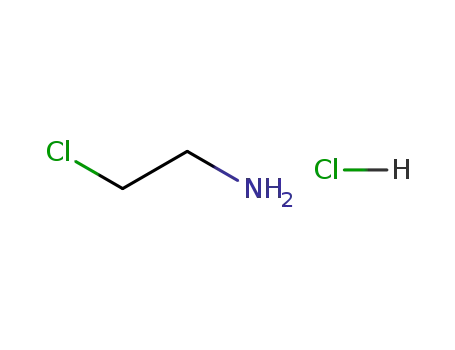
2-chloroethanamine hydrochloride

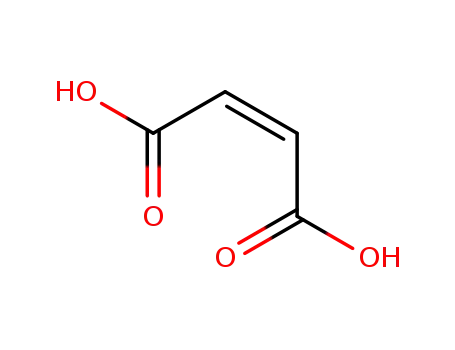
maleic acid

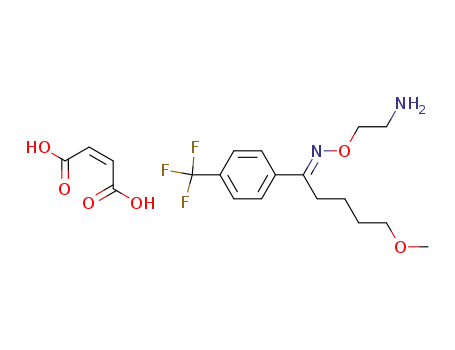
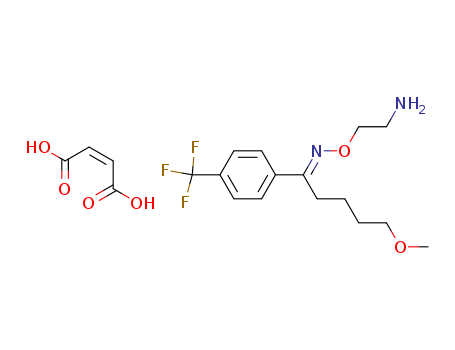
fluvoxamine maleate
| Conditions | Yield |
|---|---|
|
5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan-1-one; 2-chloroethanamine hydrochloride;
With
potassium hydroxide;
In
water; dimethyl sulfoxide; toluene;
at 40 - 45 ℃;
maleic acid;
In
water;
at 20 - 30 ℃;
|
161 mg |
![1-N-hydroxy-5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan-1-imine](/upload/2024/12/d751a323-8416-42be-8998-666bf4e7b9d2.png)
1-N-hydroxy-5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan-1-imine


2-chloroethanamine hydrochloride


maleic acid


fluvoxamine maleate
| Conditions | Yield |
|---|---|
|
With
potassium hydroxide;
In
water; toluene;
|
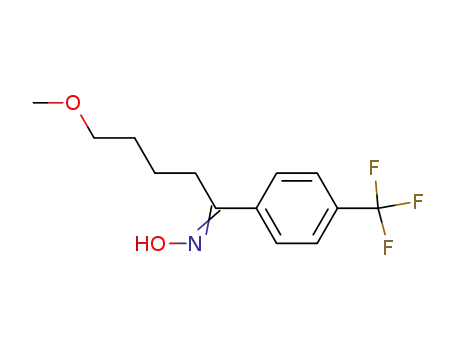
1-N-hydroxy-5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan-1-imine

2-chloroethanamine hydrochloride

maleic acid
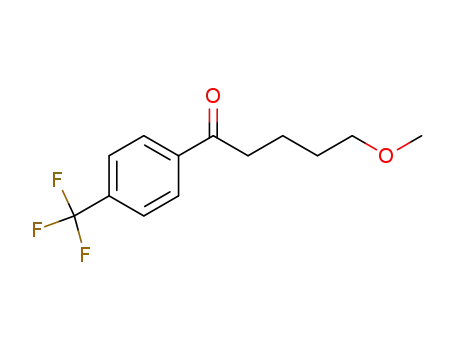
5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan-1-one
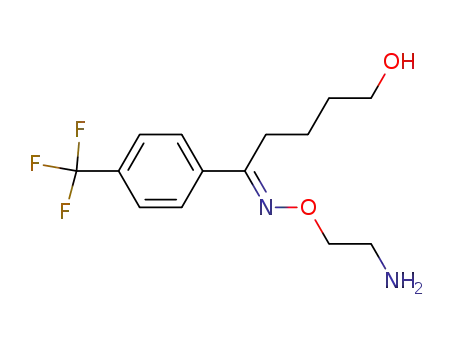
Demethyl Fluvoxamine