- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
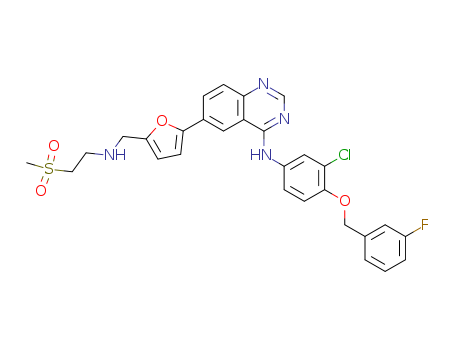
- Lapatinib
- 231277-92-2
- C<sub>29</sub>H<sub>26</sub>ClFN<sub>4</sub>O<sub>4</sub>S
- 581.067
- Powder
Your Location:Home > Products > API&Intermediates > Lapatinib

Reputable supplier selling Lapatinib 231277-92-2 with stock We supply high quality Lapatinib (CAS 231277-92-2), in stock, factory directly supply to clients, lower prices, more competitiveness.
Lapatinib is Powder, while it's Molecular Formula is C29H26ClFN4O4S. An antineoplastic agent used in breast cancer research
The CAS number of Lapatinib is 231277-92-2.
More information of Lapatinib 231277-92-2 are:
|
Synonyms |
GW572016;N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-[5-[(2-methylsulfonylethylamino)methyl]-2-furyl]quinazolin-4-amine;Lapatinib base;Lapatinib & its intermediates;Lapatinib(TINIBS);GW 572016;GSK 572016;Lapatinib [INN]; |
|
CAS Number |
231277-92-2 |
|
Molecular Formula |
C29H26ClFN4O4S |
|
Molecular Weight |
581.067 |
|
Density |
1.381 g/cm3 |
|
Boiling Point |
750.7 °C at 760 mmHg |
|
Flash Point |
407.8 °C |
|
HS CODE |
29349990 |
|
PSA |
114.73000 |
|
LogP |
7.68380 |
|
Pka |
6.34±0.19(Predicted) |
Lapatinib, a new member of the 4-anilinoquinazoline class of RTK inhibitors (RTKIs), was launched as an oral treatment for breast cancer. Lapatinib has dual affinity for EGFR and HER2 tyrosine kinases. It is indicated in combination with capecitabine for treating patients with advanced or metastatic breast cancer whose tumors overexpress HER2 and who have received prior therapy including an anthracycline, a taxane, and trastuzumab. Previously marketed drugs from the 4-anilinoquinazoline class include erlotinib (Tarceva) and gefitinib (IressaTM), both of which are indicated for treating non-small-cell lung cancer (NSCLC). As with erlotinib and gefitinib, To Market, To Market 2007 475 lapatinib is an ATP-competitive kinase inhibitor. It inhibits the tyrosine kinase activity EGFR and HER-2 with apparent Ki values of 3 and 13 nM, respectively, and has slow off-rate kinetics (t1/2X300 min). In addition, dividing the daily dose of lapatinib results in approximately 2-fold higher exposure at steady state compared to the same total dose administered once daily.The chemical synthesis of lapatinib entails the condensation of 4-chloro-6-iodoquinazoline and 3-chloro-4-(3-fluorobenzyloxy)aniline to produce a diaryl amine intermediate followed by Stille coupling of the iodo group with 5-dioxolanyl-2-(tributylstannyl)furan and subsequent acid hydrolysis of the cyclic ketal to the corresponding aldehyde. Finally, reductive amination of the aldehyde intermediate with 2-(methanesulfonyl) ethylamine in the presence of sodium triacetoxyborohydride produces lapatinib. .
InChI:InChI=1/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35)
Articles related to Lapatinib:
|
Article |
Source |
|
Unexpected Single Crystal Growth Induced by a Wire and New Crystalline Structures of Lapatinib |
De Araujo, Gabriel L.B.,Zeller, Matthias,Smith, Daniel,Nie, Haichen,Byrn, Stephen R. , p. 6122 - 6130 (2016) |
|
IMPROVED PROCESS FOR THE PREPARATION OF LAPATINIB BASE AND IT'S ANHYDROUS DITOSYLATE SALT |
- Paragraph 11, (2020/07/15) |
Hubei Mawer Biological Technology Co., Ltd. is a quality supplier and manufacturer of Lapatinib . You can buy high quality, low price Lapatinib 231277-92-2 here. Contact us.